Laboratory of Mammalian Cell Design and Engineering
Laboratory of Mammalian Cell Design and Engineering
Laboratory of Mammalian Cell Design and Engineering
Laboratory of Mammalian Cell Design and Engineering
Laboratory of Mammalian Cell Design and Engineering
GRADUATE STUDENTS


Laboratory of Mammalian Cell Design and Engineering
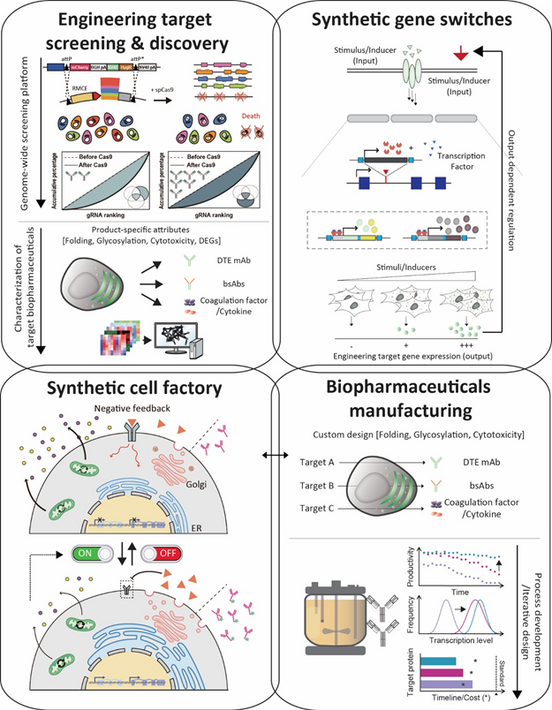
We are driving the development of next-generation mammalian cell-based production platforms to enable the efficient and consistent manufacturing of high-quality biopharmaceuticals. Our strategy combines genome editing and gene expression regulation technologies, underpinned by large-scale big data analysis of mammalian cells. By elucidating and controlling the expression mechanisms of antibodies and therapeutic proteins within mammalian systems, we strive to establish foundational technologies for advanced cell line engineering. Our ultimate vision is to lead the field of mammalian synthetic biology and pioneer transformative solutions in biopharmaceutical production.
동물세포 빅데이터 분석에 기반한 유전체 교정 및 유전자 발현 조절 기술을 통해, 고품질 바이오 의약품 생산 및 제조를 위한 동물세포 기반 생산 세포주 개발 연구를 진행하고 있습니다. 항체 및 단백질 의약품이 동물세포에서 발현되는 과정을 이해하고 조절함으로써, 차세대 바이오의약품 생산을 위한 세포주 개발 원천 기술을 확보하고, 동물세포 합성생물학 분야를 선도하는 것을 목표로 하고 있습니다.